DEXSeq
1)Introduction
DEXSeq是一种在多个比较RNA-seq实验中,检验差异外显子使用情况的方法。 通过差异外显子使用(DEU),我们指的是由实验条件引起的外显子相对使用的变化。 外显子的相对使用定义为:
number of transcripts from the gene that contain this exon / number of all transcripts from the gene
大致思想:. For each exon (or part of an exon) and each sample, we count how many reads map to this exon and how many reads map to any of the other exons of the same gene. We consider the ratio of these two counts, and how it changes across conditions, to infer changes in the relative exon usage
2)安装
if("DEXSeq" %in% rownames(installed.packages()) == FALSE) {source("http://bioconductor.org/biocLite.R");biocLite("DEXSeq")}
suppressMessages(library(DEXSeq))
ls('package:DEXSeq')
pythonScriptsDir = system.file( "python_scripts", package="DEXSeq" )
list.files(pythonScriptsDir)
## [1] "dexseq_count.py" "dexseq_prepare_annotation.py" #查看是否含有这两个脚本
python dexseq_prepare_annotation.py Drosophila_melanogaster.BDGP5.72.gtf Dmel.BDGP5.25.62.DEXSeq.chr.gff #GTF转化为GFF with collapsed exon counting bins.
python dexseq_count.py Dmel.BDGP5.25.62.DEXSeq.chr.gff untreated1.sam untreated1fb.txt #count
3) 用自带实验数据集(数据预处理)
suppressMessages(library(pasilla))
inDir = system.file("extdata", package="pasilla")
countFiles = list.files(inDir, pattern="fb.txt$", full.names=TRUE) #countfile(如果不是自带数据集,可以由dexseq_count.py脚本生成)
basename(countFiles)
flattenedFile = list.files(inDir, pattern="gff$", full.names=TRUE)
basename(flattenedFile) #gff文件(如果不是自带数据集,可以由dexseq_prepare_annotation.py脚本生成)
########构造数据框sampleTable,包含sample名字,实验,文库类型等信息#######################
sampleTable = data.frame(
row.names = c( "treated1", "treated2", "treated3",
"untreated1", "untreated2", "untreated3", "untreated4" ),
condition = c("knockdown", "knockdown", "knockdown",
"control", "control", "control", "control" ),
libType = c( "single-end", "paired-end", "paired-end",
"single-end", "single-end", "paired-end", "paired-end" ) )
sampleTable ##############构建 DEXSeqDataSet object#############################
dxd = DEXSeqDataSetFromHTSeq(
countFiles,
sampleData=sampleTable,
design= ~ sample + exon + condition:exon,
flattenedfile=flattenedFile ) #四个参数
4)Standard analysis work-flow
########以下是简单的实验设计#####
genesForSubset = read.table(file.path(inDir, "geneIDsinsubset.txt"),stringsAsFactors=FALSE)[[1]] #基因子集ID
dxd = dxd[geneIDs( dxd ) %in% genesForSubset,] #取子集,减少运行量
head(colData(dxd))
head( counts(dxd), 5 )
split( seq_len(ncol(dxd)), colData(dxd)$exon )
sampleAnnotation( dxd )
############# dispersion estimates and the size factors#############
dxd = estimateSizeFactors( dxd ) ##Normalisation
dxd = estimateDispersions( dxd )
plotDispEsts( dxd ) #图1 #################Testing for differential exon usage############
dxd = testForDEU( dxd )
dxd = estimateExonFoldChanges( dxd, fitExpToVar="condition")
dxr1 = DEXSeqResults( dxd )
dxr1
mcols(dxr1)$description
table ( dxr1$padj < 0.1 )
table ( tapply( dxr1$padj < 0.1, dxr1$groupID, any ) )
plotMA( dxr1, cex=0.8 ) #图2

To see how the power to detect differential exon usage depends on the number of reads that map to an exon, a so-called MA plot is useful, which plots the logarithm of fold change versus average normalized count per exon and marks by red colour the exons which are considered significant; here, the exons with an adjusted p values of less than 0.1

############以下是更复杂的实验设计##################
formulaFullModel = ~ sample + exon + libType:exon + condition:exon
formulaReducedModel = ~ sample + exon + libType:exon
dxd = estimateDispersions( dxd, formula = formulaFullModel )
dxd = testForDEU( dxd,
reducedModel = formulaReducedModel,
fullModel = formulaFullModel )
dxr2 = DEXSeqResults( dxd )
table( dxr2$padj < 0.1 )
table( before = dxr1$padj < 0.1, now = dxr2$padj < 0.1 )##和简单的实验设计比较
5)Visualization
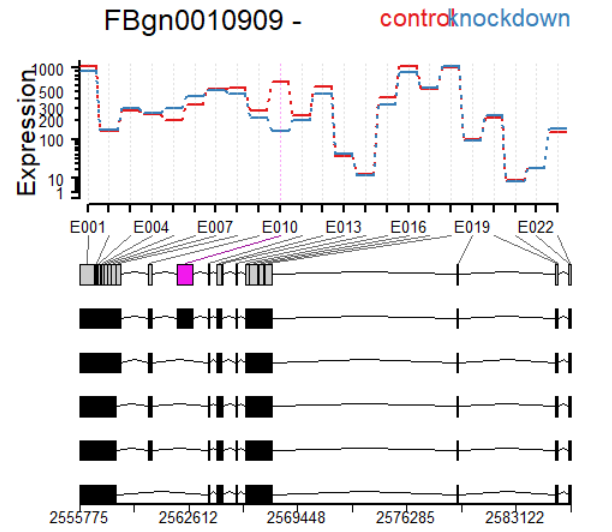
plotDEXSeq( dxr2, "FBgn0010909", legend=TRUE, cex.axis=1.2, cex=1.3,
lwd=2 )
plotDEXSeq( dxr2, "FBgn0010909", displayTranscripts=TRUE, legend=TRUE,
cex.axis=1.2, cex=1.3, lwd=2 )
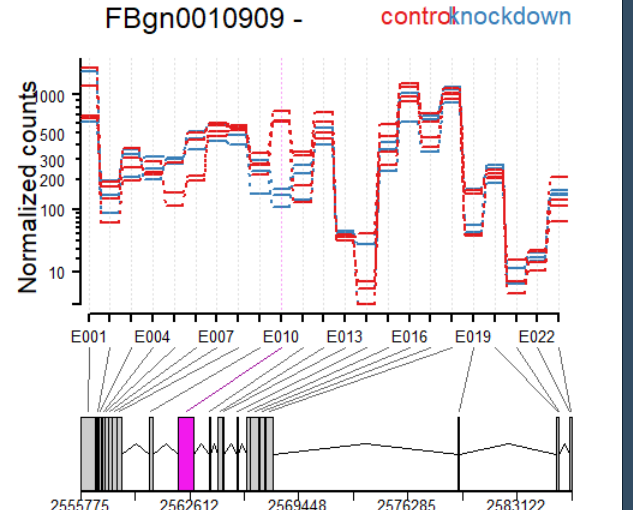
plotDEXSeq( dxr2, "FBgn0010909", expression=FALSE, norCounts=TRUE,
legend=TRUE, cex.axis=1.2, cex=1.3, lwd=2 )
plotDEXSeq( dxr2, "FBgn0010909", expression=FALSE, splicing=TRUE,
legend=TRUE, cex.axis=1.2, cex=1.3, lwd=2 )
DEXSeqHTML( dxr2, FDR=0.1, color=c("#FF000080", "#0000FF80") )


DEXSeq的更多相关文章
- 【转录组入门】6:reads计数
作业要求: 实现这个功能的软件也很多,还是烦请大家先自己搜索几个教程,入门请统一用htseq-count,对每个样本都会输出一个表达量文件. 需要用脚本合并所有的样本为表达矩阵.参考:生信编程直播第四 ...
- Bulk RNA-Seq转录组学习
与之对应的是single cell RNA-Seq,后面也会有类似文章. 参考:https://github.com/xuzhougeng/Learn-Bioinformatics/ 作业:RNA-s ...
- Bioconductor应用领域之基因芯片
引用自https://mp.weixin.qq.com/s?__biz=MzU4NjU4ODQ2MQ==&mid=2247484662&idx=1&sn=194668553f9 ...
随机推荐
- 在VS2008中加入ExtJS智能提示
在VS2008中加入ExtJS智能提示 在VS2008中加入ExtJS智能提示—>(方法一) 关于如何在VS2008中加入ExtJS的智能提示的方法,我这里有2种方法,相对于第二种方法,第一 ...
- php对象的实现
1.对象的数据结构非常简单 typedef struct _zend_object zend_object; struct _zend_object { zend_refcounted_h gc; / ...
- linux常用命令解析
linux下一些注意事项 1. linux下严格区分大小写 ls 简述:列出文件或目录列表. -> ls 默认列出当前目录下的所有文件. -> ls -l(long)以长格式查看文件. - ...
- 详解 Facebook 田渊栋 NIPS2017 论文:深度强化学习研究的 ELF 平台
这周,机器学习顶级会议 NIPS 2017 的论文评审结果已经通知到各位论文作者了,许多作者都马上发 Facebook/Twitter/Blog/ 朋友圈分享了论文被收录的喜讯.大家的熟人 Faceb ...
- Bogart BogartPublic.vb
Imports System.Data.SqlClient Imports System.Data #Region "IBogartToolbar,請勿隨便更改" Interfac ...
- 杂: PYTHON上数据储存:推荐h5py
一篇很短的小短文,主要推荐下做科学计算是大量数据的储存问题 最近在做一个CNN的项目,文件夹里有20w张图片要读入并保存到一个data文件(不然每次都读20w文件太麻烦). 折腾了一个下午,发现了一个 ...
- spring boot + jpa + kotlin入门实例
spring boot +jpa的文章网络上已经有不少,这里主要补充一下用kotlin来做. kotlin里面的data class来创建entity可以帮助我们减少不少的代码,比如现在这个User的 ...
- 管道| , <<<重定向
https://blog.csdn.net/stormbjm/article/details/19173011
- pythone--002
元组就是不可修改: 字典的索引不是自增的. 元组和列表是: 默认 是key 通过get 没有这个key 是none get可以有默认值: 通过索引 没有就报错. 检查字典中某个可以是否存在:ha ...
- sbt的安装测试
1.下载 wget https://github.com/sbt/sbt/releases/download/v0.13.15/sbt-0.13.15.tgz 2.安装 tar -zxvf sbt-0 ...
